New Alzheimer’s Drug May be the Breakthrough They Need.
Medical professional’s are hopeful after a new drug, Lecanemab shows promising results on treating Alzheimer’s.
curonal brain,brain tumor,blood brain barier
December 1, 2022
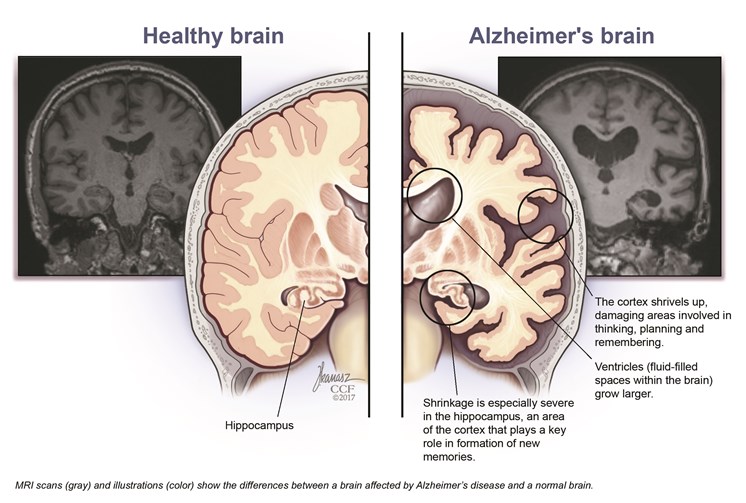
New Alzheimer’s medication may just be the biggest breakthrough in treating the disease in years. After years of research leading to a stalemate and failures, people working in the medical field are ecstatic after a new drug known as Lecanemab has been show to slow to progress of the mental disease. The drug is able to reduced the amount of brain amyloid’s, and in doing so decreases the cognitive declines in patients. It does this by binding to amyloid beta which is extremely toxic to the brain clumps together to form plaque that collects between neurons and disrupts cell functions. In doing so it Deceases the amount significantly.
While trials in phase two patients yielded no different results from a placebo drug. In phase three patients shows that those around the 18 month point of the trials the drug was around twenty-seven percent effective at helping reducing the decline in mental capabilities. Based on the research doctors are hopeful for the potential of the drug, as it has been shown to help patients in retaining their cognitive functions. This is such an amazing advancement within the medical industry especially given before this drug, there was no chance of peoples with Alzheimers retaining any information and giving hope to families to have their beloved relatives remember who they are.
It’s not all good however, as multiple questions have arisen of the long-term effects and safety related around the drug. As the statistics show us 14% of those taking Lecanemab experienced adverse effects compared to the 11.3% of the placebo effect, with 6.9 % of the patients taking the new drug being discontinued do to it’s effects while only 2.9% of the those taking the placebo were discontinued. Most of these adverse effects included brain swelling and bleeding in the brain, this was mostly due to ARIA which stands for amyloid related imaging abnormalities. The statistics on these show that a staggering 17.3% of those who received Lecanemab have been shown to have ARIA brain bleeding to only 9% of those in the placebo group. 12.6 of those who take the experimental drug have experienced brain swelling to the 1.7% of the placebo group. However only 0.7 of those in the Lecanemab group died compared to the 0.8% and none of the deaths were correlated with Lecanemab or ARIA issues.
Despite the draw backs shown medical professionals are excited for the future developments of the drug and are expecting the FDA to decide the approval of Lecanemab by the 6th of January, 2023 as long as the trials show that the drug produces a clinical benefit in patients. Overall those in the field of treating the mental disease are watching the drug closely as the Alzheimer’s scene has been “starving” for development for years.